Virus:SARS-COV2
Disease: COVID19
In order for us to understand how the SARS-COV2 interacts with drugs, we must review some basic principles of Molecular biology.
Bridge to molecular biology – Background overview
- Instructions to make proteins are contained into our DNA
- DNA contains genes, which are strings of nucleotides containing a region that codes for an RNA molecule
- This region begins with a recognition site, known as, the promoter region and a termination site, known as the termination region
- Genes also contain regulatory sequences that are usually found near the promoter region.
Gene expression
- The process by which the hereditary information in a gene, is made into a functional product, such as protein or RNA.
- The basic idea is that DNA is transcribed into RNA, which is then translated into proteins
- A particular segment of DNA is copied into RNA (especially mRNA) by the enzyme RNA polymerase:
- If the gene encodes a protein, the transcription produces messenger RNA (mRNA)
- The mRNA, in turn, serves as a template for the protein’s synthesis through translation.
Transcription- No action of SARS COV2 here

Transcription overview:
Initiation
Initiation is the beginning of transcription. It occurs when the enzyme RNA polymerase binds to a region of a gene called the promoter. This signals the DNA to unwind so the enzyme can ‘‘read’’ the bases in one of the DNA strands. The enzyme is now ready to make a strand of mRNA with a complementary sequence of bases.
Elongation
Elongation is the addition of nucleotides to the mRNA strand. RNA polymerase reads the unwound DNA strand and builds the mRNA molecule, using complementary base pairs. There is a brief time during this process when the newly formed RNA is bound to the unwound DNA. During this process, an adenine (A) in the DNA binds to uracil (U) in the RNA.
Termination
Termination is the ending of transcription and occurs when RNA polymerase crosses a stop (termination) sequence in the gene. The mRNA strand is complete, and it detaches from DNA.
Transcription steps
- RNA polymerase, with the help of transcription factors, binds to promoter DNA, This is where most of the gene expression is controlled, by allowing the binding or blocking of RNA polymerase to the promoter region.
- RNA polymerase-> breaks H-bonds between complementary DNA nucleotides ? separates the two strands of the DNA helix ? transcription bubble.
- RNA polymerase adds the complementary nucleotides (RNA) ? RNA sugar-phosphate backbone.
- Hydrogen bonds of the RNA–DNA helix, free the newly synthesized RNA strand.
Transcription modification
- Polyadenylation
- Begins with the termination of transcription
- The 3? segment of the newly synthesized mRNA is cleaved off by a set of proteins ? synthesis of the poly(A) tail at the RNA’s 3? end ? strand of RNA consisting of only adenine base
- The poly-A tail makes the RNA molecule more stable and prevents its degradation and allows the mature messenger RNA molecule to be exported from the nucleus and translated into a protein by ribosomes in the cytoplasm.
- The tail is shortened over time, and, when it is short enough, the mRNA is enzymatically degraded
- Capping
- 5′ capping is essential for mRNA stability, enhancing mRNA processing, mRNA export and translation.
- After capping, phosphorylation initiates RNA splicing
- It is the transformation of a newly made precursor messenger RNA (pre- mRNA) into a mature messenger RNA (mRNA).
- During splicing, introns (Non-coding regions) are removed and exons (Coding Regions) are joined together by spliceosomes
Translation SARS COV2 acts here
- Occurs in the cytoplasm
- It is the process in which ribosomes in the cytoplasm or ER synthesize proteins after the process of transcription of DNA to RNA in the cell’s nucleus
Overview:
- Initiation
- The ribosome assembles around the target mRNA.
- The first tRNA is attached at the start codon
- Elongation
- The tRNA transfers an amino acid to the tRNA corresponding to the next codon.
- The ribosome then moves to the next mRNA codon to continue the process, creating an amino acid chain.
- Termination
- When a peptidyl tRNA encounters a stop codon, then the ribosome folds the polypeptide into its final structure.
Translation steps- Notes marked in red indicate action in case of SARS2 infection
- mRNA is decoded in the ribosome to produce a specific amino acid chain, or polypeptide. .
- Nitrogenous groups are paired into 3 letter groups, known as codones
- The genetic code is composed of 64 codones
- Most codones code for specific Amino Acids (AA)
- There are 4 special codones: 1 for starting and 3 for termination
- AUG: Start codone
- UGA: Stop codone
- UAG: stop codone
- UAA: stop codone
- Each AA is brought to the ribosome by a specific tRNA molecule
- Complementary base pairing occurs between codone of mRNA and anticodone of tRNA
- Translation begins when mRNA binds to a ribosomal unit, upstream of the start codone (AUG)- Host’s ribosome will attach to SARS COV2 RNA
- Large ribosome subunits, contain 3 sites: E,A,P
- Base pairing continues to occur between codone of mRNA and anticodone of tRNA
- A charged tRNA binds to the A-site and a peptide bond is formed between its AA and its neighboring AA at the P-site, leaving an uncharged molecule at the P-site
- Complex slides one codone to the right
- The uncharged molecule at the P-site, exists the complex at the E-site, leaving room for another molecule to join
- Now tRNA at A-site is ready to accept the next tRNA molecule
- Elongation will continue until a stop codone is reached
- A release binds to A-site at a stop codone
- Polypeptide is released from tRNA at the P-site- Polypeptide released is RNA-dependent RNA Polymerase (RdRP)
- Polypeptide may need further modifications at various organelles, before it is ready to function


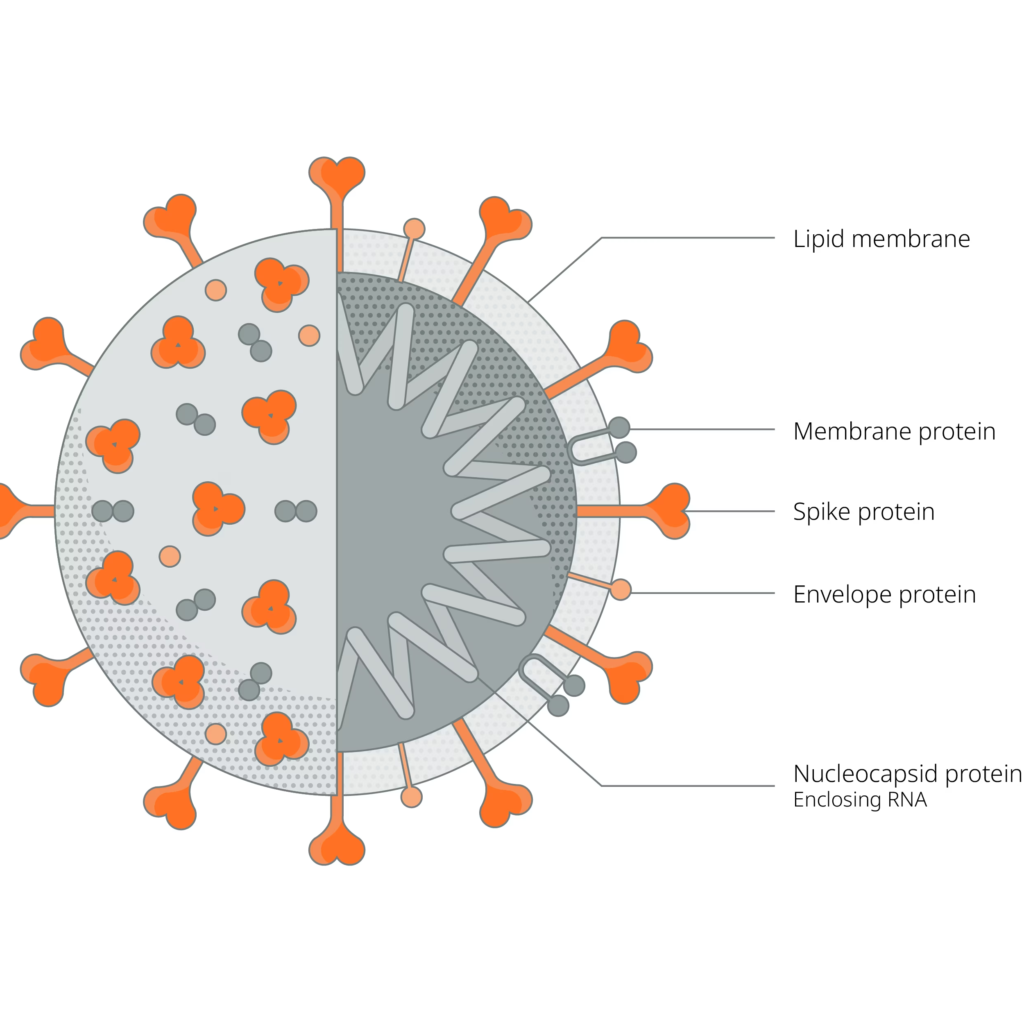
- As you can see from the diagram above, The virus attaches to the ACE-2 Receptor of the host cell and injects its own RNA into the cytoplasm of the cell
- Now the host’s ribosomes will attach to the viral RNA and will start the translation process? producing a new protein : RNA-dependent RNA Polymerase (RdRP)
- RNA-dependent RNA Polymerase (RdRP)
- RdRP will attach to the polyA tail and will start the translation process ? identical copy of original viral genone (RdRP copy)
- Somtimes the RdRP protein stops synthesis early? subgenomic RNA’s (SgRNA), which are shorter pieces of original copy
- Subgenomic RNA’s are again translated by host’s ribosomes? viral proteins
- Viral reassemble into a new virus,and released from the cell, ready to infect another person
- Zinc has been found to block the RdRP but it requires an ionophore (a substance which is able to transport particular ions across a lipid membrane in a cell)
- Chloroquine might be effective because it is a zinc ionophore
The controversy of ACE2i & ARBs
- SARS-cov2 binds to ACE2 receptor with the help of Serine protease
Theory1: ACEi worsens outcomes of SARS COV2 patients:
- ACE inhibitors are believed to increase the ACE2 receptors, and serine protesases (TMPRSS2)
- Since SARS binds to the ACE2 receptors, it is suggested that ACEi might worsen the prognosis of COVOD19 patients
Theory2: ACEi improve the outcome of SARS COV 2 patients
- Behavior of AT2 levels theories
In a low level state
Since there isn’t enough AT2 around, it is proposed that Angiotensin Receptor1(ATR1) binds to the catalytic site of ACE2R forming a complex? release of Angiotensin 1,7 ? Activating MAS receptor ? health benefits listed in the diagram (Green box)
- It is believed that ARBS keep the ATR1-ACE2 complex together, leading to the release of Angiotensin 1,7
In a high level state
AT2 breaks the ATR1-ACE2 complex, exposing the ACE2 receptor causing:
- SARS COV2 to bind to ACE2, leading infection
- There is currently an ongoing experiment about blocking the serine protease protein (TMPRSS2), which allows SARS COV2 to bind to ACE2, by the action of Camostat methylate
- Some studies suggest that the ACE/SARS COV2 complex gets degraded by lysosomes
- AT2 binds to the AT1 receptor, leading to health issues listed in the diagram (red box), which is the main cause of health issues, even if the virus gets destroyed or is unable to bind.
A study in mice ( ACE2 knockout mice), with deleted AC2 receptors, showed
- Resistance to infection by SARS (binds to same receptor as SARS COV2)
- Low cardiac contractility
- Poor prognosis with viral pneumonia
- Increased levels of AT2
These outcomes are seen in current SARS COV2 patients
Another study in 2002, on mice infected with SARS, showed improved outcomes when these mice were given ARBS and ACEi
The European society said that we don’t have enough evidence to advise the cease of prescribing ACEi or ARBS, at this point and animal studies indicate that such drugs might improve the outcome.
The American college of cardiology issued a positioning statement in the matter saying “The continued highest standard of care for cardiovascular disease patients diagnosed with COVID-19 is the top priority, but there are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-I or ARB medications,” said Richard J. Kovacs, MD, FACC. “We urge urgent, additional research that can guide us to optimal care for the millions of people worldwide with cardiovascular disease and who may contract COVID-19.
Further research is needed, however, there is currently no sufficient evidence against the use of ACEi and ARBs for hypertensive patients.
Another study in 2002, on mice infected with SARS, showed improved outcomes when these mice were given ARBS and ACEi
The European society said that we don’t have enough evidence to advise the cease of prescribing ACEi or ARBS, at this point and animal studies indicate that such drugs might improve the outcome.
The American college of cardiology issued a positioning statement in the matter saying “The continued highest standard of care for cardiovascular disease patients diagnosed with COVID-19 is the top priority, but there are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-I or ARB medications,” said Richard J. Kovacs, MD, FACC. “We urge urgent, additional research that can guide us to optimal care for the millions of people worldwide with cardiovascular disease and who may contract COVID-19.
Further research is needed, however, there is currently no sufficient evidence against the use of ACEi and ARBs for hypertensive patients.